Our mission
We utilize our remote patient monitoring platform to capture real-world longitudinal EEG data. Our Big Data streams are transformed by stakeholders such as physicians and drug developers into actionable insights intended to personalize treatment and reduce uncontrolled seizures in epilepsy. Through partnerships with these stakeholders, we solve neurological challenges and unlock new possibilities in treatment, drug development, digital biomarkers and neuromodulation – driving brain health therapy forward to help patients live a better life.
Longitudional real-world EEG data

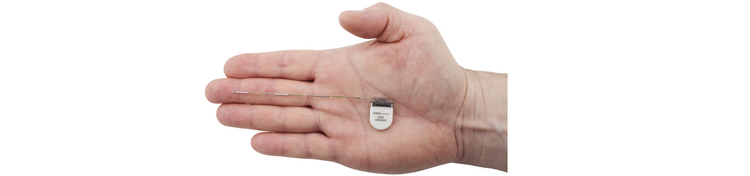
UNEEG EpiSight System
UNEEG EpiSight System is our breakthrough solution in epilepsy management - one that enables continuous, subcutaneous EEG recording for years. Currently, we have collected more than 400,000 hours of data. It’s a paradigm shift that gives healthcare professionals the truly objective, longitudinal seizure and sleep data needed to address challenging diagnostic questions and track seizure burden - and help patients live a better life.

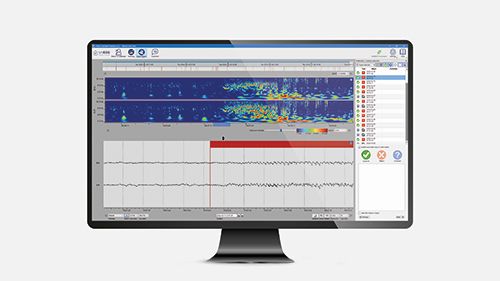
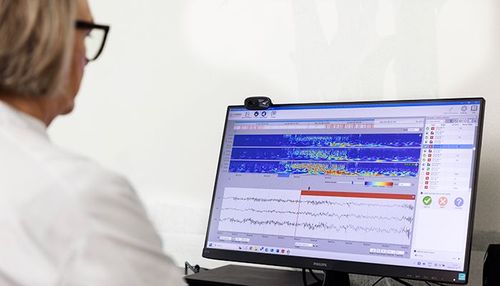
UNEEG EpiSight Analyzer
Introducing a new sleep analysis module in UNEEG EpiSight Analyzer which allows health care professionals to correlate sleep and seizure data. Exploring the well-known bi-directional link between epilepsy and sleep, and evaluating the impact of treatment. Longitudinal EEG data for up to 3 years is a new powerful tool in epilepsy management.
Why UNEEG EpiSight and real-world longitudinal EEG?
Around 40% of people with epilepsy continue to suffer from uncontrollable seizures, despite trying at least two anti-seizure medications. In fact, many of them exhaust standard-of-care diagnostic pathways only to remain with unresolved diagnostic gaps and issues. UNEEG EpiSight brings new insights to such uncertainties with longitudinal, objective data recorded during patients’ everyday lives.

UNEEG EpiSight solution
A discreet, unobtrusive solution designed for real-world life. It enables continuous EEG recording carried out as patients live their normal lives. The solution comprises the following elements:
UNEEG EpiSight Recorder
Designed for real-world life, discreet and wireless. Retrieves and transfers raw EEG data and self-reported seizures to the hospital. 24 hours-plus battery and storage capacity.
UNEEG EpiSight App
For users' smart phones to manage the recorder and register seizures. Easy and intuitive interface.
UNEEG EpiSight Analyzer
Our proprietary AI Analytics Software platform analyses the EEG recording in minutes. It identifies suspected seizure activity for fast review, and correlates with sleep overviews. Both raw and analysed data available at your convenience.
Unique opportunities with ultra long-term EEG recording
For epilepsy management
Address remaining diagnostic uncertainties.
Optimise pathway to the best possible balance between efficacy and side effects.
Longitudinal data correlation in summarised overviews.
Put patients’ lived experiences at the centre of epilepsy care.
Support dialogue and selfmanagement efforts with objective insights.
Unobtrusive, non-stigmatising look and feel promotes patient acceptance. Easy user interface supports good patient adherence.
Longitudinal and objective data - distilled and delivered to your desktop
At the end of each day, the UNEEG EpiSight Analyzer AI software sifts through the recorded data and in minutes highlights suspected epileptic seizure activity for fast review at your convenience. A concise EEG report with summarised results can be generated. Healthcare professionals have easy, on-demand access to results and all the recorded raw data, and can match relevant time periods to see the effects of management changes.
The AI analytics software makes it possible to:
- Identify the seizure burden over time
- View daily seizure distribution
- Compare the seizure activity over two time periods
- Compare recorded seizure activity with patient-reported events
- Get insights about sleep, including total sleep time and daytime naps
- Identify patterns between seizures and specific sleep stages
Monitoring epilepsy with subcutaneous EEG
Murray shares his personal story with epilepsy and what it is like to live with the 24/7 EEG SubQ which records his EEG in daily life and monitors his epilepsy.
Benjamin's epilepsy is monitored 24 hours a day, 7 days a week with the 24/7 EEG SubQ. In this video he explains how he and his neurologist have used the data obtained from the 24/7 EEG SubQ to improve his quality of life.
Highlights
UNEEG Medical marks first pediatric implantation in Europe-led study
1/8/2026
COPENHAGEN, January 8, 2026 – UNEEG Medical is proud to announce a groundbreaking milestone: for the first time, a child has been included in a clinical study using UNEEG’s ultra long-term brain monitoring solution.
UNEEG medical Achieves EU Approval for 3-Year Continuous Use of UNEEG SubQ Implant – Further Strengthening its EpiSight Solution for Epilepsy Monitoring
6/11/2025
COPENHAGEN, June 11, 2025 – UNEEG medical, a Danish company at the forefront of neuro- and epilepsy science, proudly announces the EU approval for 3-year continuous use of its subcutaneous implant, which is a significant milestone for the UNEEG EpiSight solution.
New CEO at UNEEG medical
3/7/2025
Martin Stenfeldt has taken over as new CEO of UNEEG medical. He succeeds Torben Sandgren, who has chosen to resign from his position after 7 years of dedicated service.
Contact
UNEEG medical
Borupvang 2
DK-3450 Allerød
Denmark
Phone: +45 4063 8000
Mail: uneeg@uneeg.com
VAT-NO: DK29140774
CVR-NO: 29140774
Contact us